What is “PPM”?
The ppm is an acronym for “parts per million”, a unit of measure that describes how much of one substance (called the “solute”) is dissolved in a sample of water (called the “solvent”). This is a measurement of concentration (or density), which can often be helpful in our daily lives. The ppm can be used to measure the concentration of many substances, such as the minerals in our drinking water or the oxygen in a fish aquarium. Although the ppm is commonly used for concentration measurements, it only provides us with a ratio of the solute’s mass to the mass of the water, without specifying the water’s total volume. But, more often we need to know the total amount of solute dissolved in the water.
This is extremely important, for example, when determining the therapeutic dose for a medicine or the ingested level of a toxin. Without including the size of the container we are testing, which tells us the amount of water our solute is dissolved in, the ppm ratio by itself does not tell us how much solute the water contains. To convey this information, scientists use a more appropriate unit of measure, one which specifies the solute’s concentration in units of “mass per unit volume”. The unit commonly used is the “milligram per liter”, abbreviated mg/L. The mg/L always references the solute’s mass relative to a fixed volume, one liter.
Note: Frequently, the ppb (parts per billion) is used to measure solute concentration. Although the ppb is not used in this article, 1 ppm = 1000 ppb
How are ppm and mg/L related?
We can think of 1 ppm as “1 part of a substance (solute) dissolved in 1 million parts of a solution” (in our case the solute is H2, hydrogen gas, and the solution is water). So, what is a “part”? “part” represents a unit of measure, in our case the “milligram” (mg). Therefore, 1 milligram of H2 (“1 part”) dissolved in 1 million milligrams of water (“1 million parts”) is “one part per million”. And, since 1 liter of water happens to weigh 1 million milligrams, 1 ppm is equal to 1 mg/L (for dilute concentrations).
Note: This is only true when comparing units of mass, not when comparing volumes, # of moles, or # of molecules.
Calculating the amount of ingestible H2 based on the concentration:
If the concentration is given in ppm, first convert the ppm to mg/L. Then, multiply the volume of water (in liters) by the concentration (in mg/L) to calculate the amount of H2 which will be ingested (in mg). When considering ingested amounts, not only the concentration, but also the volume of ingested water must be considered. This is because it takes fewer milligrams of H2 to produce 1 ppm in a smaller amount of water than it does in a larger volume of water. The graphic below illustrates this concept:
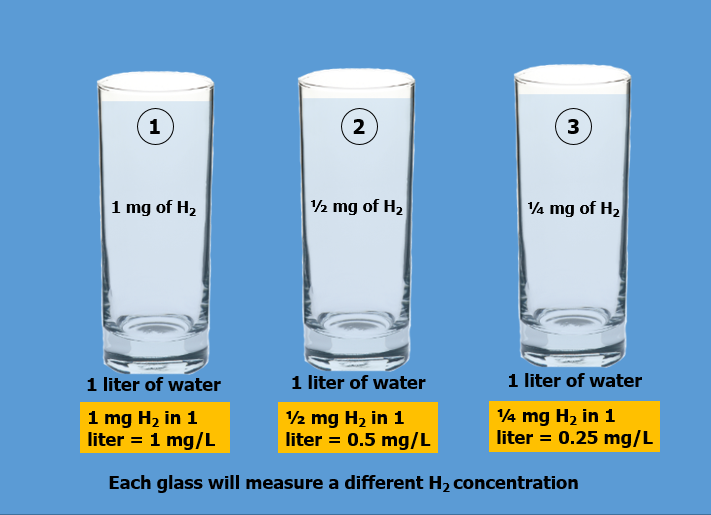
In the image above, it shows 3 glasses, each of which contains the same amount of water, 1 liter, and also the same three amounts of dissolved H2 as in first image, 1mg, 0.5 mg & 0.25 mg. Now, in contrast to first image, they will each measure different concentrations of H2, 1 mg/L, 0.5 mg/L and 0.25 mg/L respectively. Just as in Figure 1, the three glasses in this example do not contain the same amount of dissolved H2. And, because their concentrations are different, drinking the entire contents of each glass WILL NOT provide the same levels of ingested H2. In order to receive the same ingested amounts of H2, you will need to drink varying amounts of each as follows:
Glass #1: You must drink 1 liter of 1 mg/L water to ingest 1 milligram of H2
Glass #2: You must drink 2 liters of 0.5 mg/L water to ingest 1 milligram of H2
Glass #3: You must drink 4 liters of 0.25 mg/L water to ingest 1 milligram of H2
From this example, it can be seen that when the ppm reading is referenced to a volume of 1 liter (expressed in terms of “mg/L”), the measured concentration in mg/L DOES indicate how much H2 will be ingested when drinking 1 liter from each container.
The following example will help to illustrate why the mg/L measurement alone cannot tell us how much dissolved H2 a sample of water contains. Image below shows the results of dissolving the same amount of H2 into three different volumes of water, 1 liter, ½ liter, and ¼ liter.

If the same amount of H2 gas is dissolved into half the volume of water, the measured H2 concentration (in mg/L) will double. But, this does not mean that the amount of dissolved H2 has doubled-in fact, the amount of H2 has remained the same. Therefore, as we have seen in these examples, to know how much H2 will be consumed, both the concentration and the volume of water must be considered.
Summary:
The ppm is a unit of measurement which provides a ratio of the amount of a dissolved substance to the amount of water it is dissolved in. Therefore, 1 milligram of a substance dissolved in 1 million milligrams of water will have a concentration of “1 part per million”. Because 1 liter of water weighs 1 million milligrams, 1 part per million is also the same as 1 milligram per liter when referenced to a volume of one liter. Because flow-through type devices are generally capable of producing a liter or more of water having the same H2 concentration, the measured concentration of the water from this class of device will typically be representative of the number of milligrams of H2 we will ingest if we drink one liter.
When interpreting the measurement from a sample of H2 water produced by technologies other than flow-through type devices (tablets, sticks, cartridges, etc.), the ppm measurement must be converted to mg/L by adjusting it to the volume of H2 water being sampled. Only by performing this conversion can we determine how much H2 we will ingest when drinking the entire contents of the sampled container.
Source: https://molecularhydrogeninstitute.org/calculating-the-dose-of-h2/
















